How To Find Charge Of Cation And Anion
iii.3: Predicting Charges of Ions
- Page ID
- 217255
Skills to Develop
- Define ions, cations and anions
- Predict the charge of an anion or cation based upon the location of the chemical element in the periodic tabular array
Ions of Elements
In ordinary chemical reactions, the nucleus of each atom remains unchanged. Electrons, yet, can be added to atoms by transfer class other atoms, lost past transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemical science of the elements. During the formation of some compounds, atoms proceeds or lose electrons, and form electrically charged particles called ions (Figure \(\PageIndex{ane}\); Video \(\PageIndex{one}\))
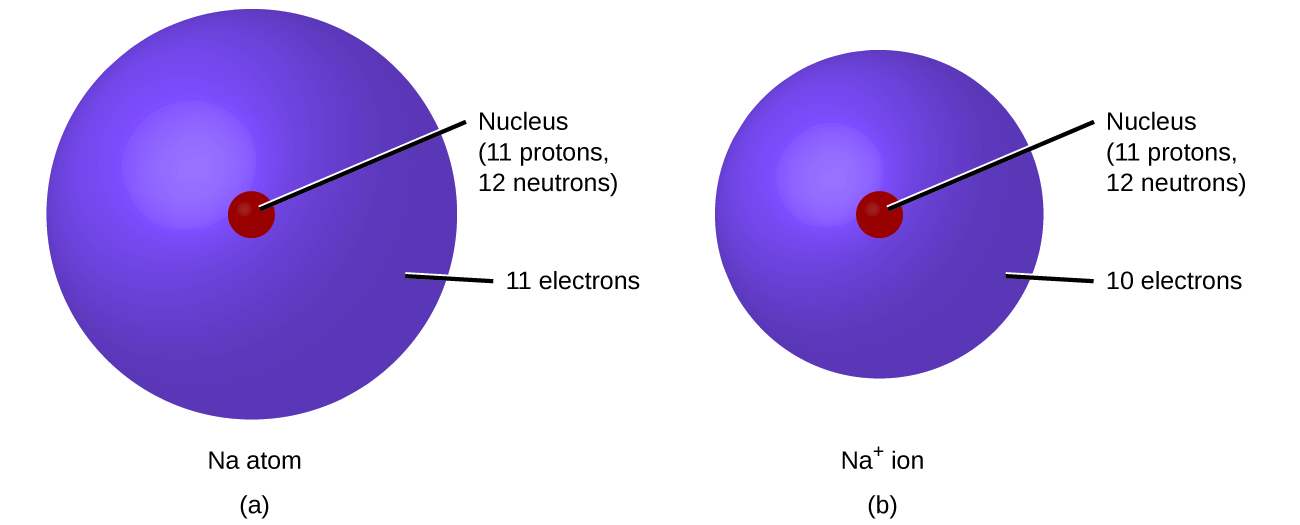
Effigy \(\PageIndex{ane}\): (a) A sodium atom (Na) has equal numbers of protons and electrons (11) and is uncharged. (b) A sodium cation (Na+) has lost an electron, so information technology has one more proton (xi) than electrons (ten), giving information technology an overall positive charge, signified by a superscripted plus sign.
One can use the periodic table to predict whether an cantlet will grade an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-grouping metals lose enough electrons to leave them with the aforementioned number of electrons as an atom of the preceding noble gas. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; an alkaline earth metal (group ii) loses ii electrons and forms a cation with a ii+ charge, and so on. For case, a neutral calcium atom, with 20 protons and twenty electrons, readily loses two electrons. This results in a cation with 20 protons, 18 electrons, and a 2+ accuse. It has the aforementioned number of electrons as atoms of the preceding noble gas, argon, and is symbolized Ca2+. The name of a metal ion is the aforementioned as the proper noun of the metal atom from which it forms, so Caii+ is called a calcium ion.
When atoms of nonmetal elements grade ions, they generally gain plenty electrons to give them the same number of electrons as an atom of the next noble gas in the periodic tabular array. Atoms of group 17 gain 1 electron and class anions with a one− accuse; atoms of group sixteen proceeds two electrons and form ions with a 2− accuse, and so on. For example, the neutral bromine cantlet, with 35 protons and 35 electrons, tin can gain one electron to provide it with 36 electrons. This results in an anion with 35 protons, 36 electrons, and a ane− accuse. It has the aforementioned number of electrons as atoms of the next element of group 0, krypton, and is symbolized Br−. (A give-and-take of the theory supporting the favored status of noble gas electron numbers reflected in these predictive rules for ion formation is provided in a after chapter of this text.)
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure \(\PageIndex{2}\)). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the grouping number. That is, group 1 elements class one+ ions; group two elements form 2+ ions, and so on. Moving from the far right to the left on the periodic table, elements often course anions with a negative charge equal to the number of groups moved left from the noble gases. For example, grouping 17 elements (one group left of the noble gases) grade 1− ions; grouping 16 elements (two groups left) form 2− ions, and so on. This trend tin be used equally a guide in many cases, only its predictive value decreases when moving toward the eye of the periodic table. In fact, transition metals and some other metals often exhibit variable charges that are not predictable past their location in the tabular array. For instance, copper tin can form ions with a 1+ or two+ charge, and fe tin can form ions with a 2+ or 3+ charge.
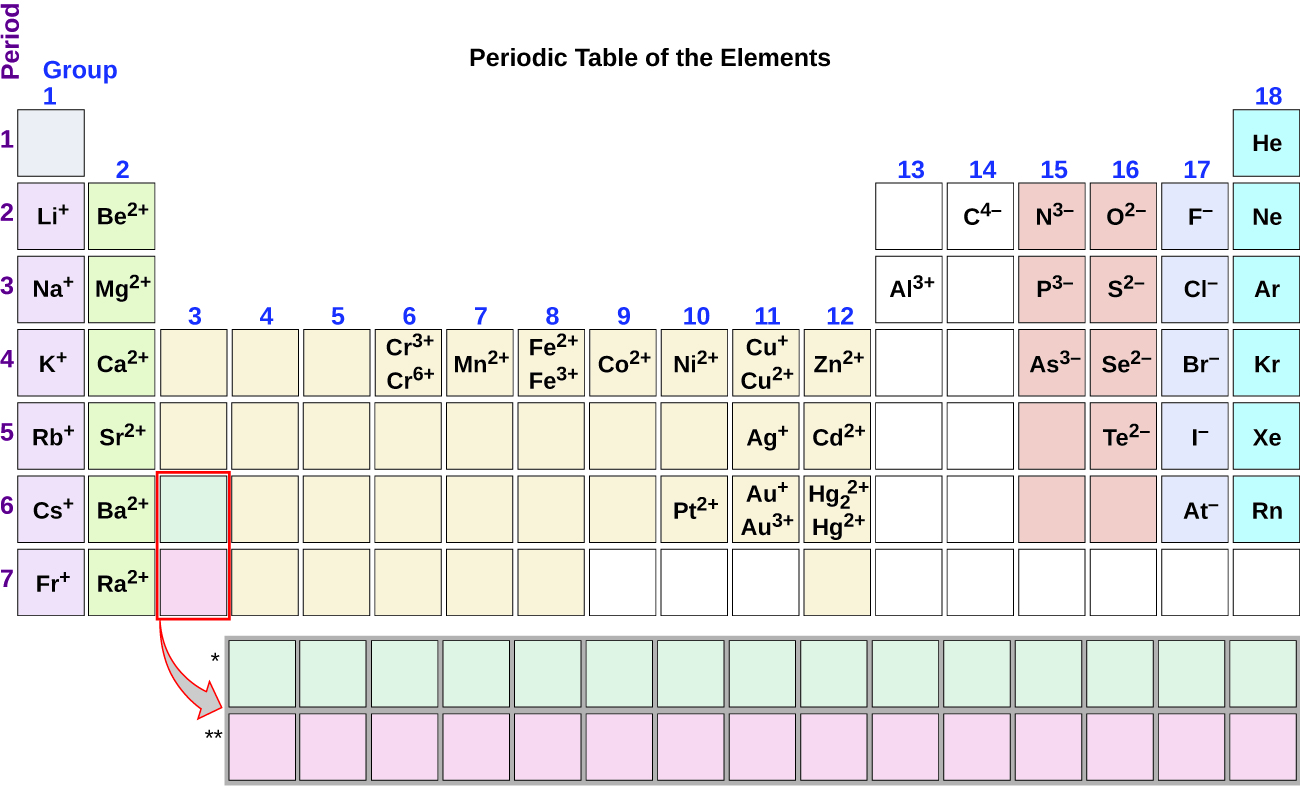
Figure \(\PageIndex{2}\): Some elements exhibit a regular pattern of ionic charge when they form ions.
Example \(\PageIndex{one}\): Composition of Ions
An ion found in some compounds used as antiperspirants contains thirteen protons and x electrons. What is its symbol?
Solution
Considering the number of protons remains unchanged when an atom forms an ion, the diminutive number of the chemical element must be 13. Knowing this lets u.s.a. apply the periodic tabular array to identify the element as Al (aluminum). The Al cantlet has lost three electrons and thus has three more positive charges (13) than it has electrons (10). This is the aluminum cation, Althree+.
Exercise \(\PageIndex{one}\)
Requite the symbol and proper noun for the ion with 34 protons and 36 electrons.
- Answer
-
Se2−, the selenide ion
Instance \(\PageIndex{2}\): Formation of Ions
Magnesium and nitrogen react to class an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and proper name them.
Solution
Magnesium's position in the periodic table (group 2) tells the states that information technology is a metallic. Metals course positive ions (cations). A magnesium atom must lose two electrons to accept the aforementioned number electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a accuse of ii+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Nitrogen's position in the periodic table (group 15) reveals that it is a nonmetal. Nonmetals form negative ions (anions). A nitrogen cantlet must proceeds iii electrons to accept the same number of electrons equally an cantlet of the following noble gas, neon. Thus, a nitrogen cantlet will form an anion with iii more electrons than protons and a charge of three−. The symbol for the ion is Niii−, and information technology is chosen a nitride ion.
Exercise \(\PageIndex{ii}\)
Aluminum and carbon react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and proper name them.
- Answer
-
Al will form a cation with a charge of 3+: Aliii+, an aluminum ion. Carbon will form an anion with a charge of four−: C4−, a carbide ion.
Summary
Video \(\PageIndex{1}\): What are ions?
Glossary
- anion
- negative ions that are formed when a nonmental atom gains one or more electrons.
- cation
- positive ions that are formed when an cantlet loses electrons
- ion
- cantlet or molecule that has gained or lost i or more of its valence electrons, giving it a cyberspace electrical charge.
- Contributors
-
Paul Flowers (University of Due north Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors.Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License four.0 license. Download for gratuitous at http://cnx.org/contents/85abf193-2bd...a7ac8df6@ix.110).
- Adelaide Clark, Oregon Institute of Engineering science
- Fuse School, Open Educational Resource free of charge, under a Creative Commons License: Attribution-NonCommercial CC By-NC (View License Human activity: https://creativecommons.org/licenses/by-nc/4.0/)
Source: https://chem.libretexts.org/Courses/Oregon_Tech_PortlandMetro_Campus/OT_-_PDX_-_Metro%3A_General_Chemistry_I/03%3A_Nuclei_Ions_and_the_Periodic_Table/3.03%3A_Predicting_Charges_of_Ions
Posted by: kellerflust1973.blogspot.com

0 Response to "How To Find Charge Of Cation And Anion"
Post a Comment